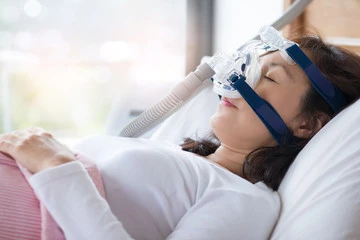
While continuous positive airway pressure (CPAP) is the most common treatment for sleep apnea, 50-80% of patients fail this modality in the first year. Other options, such as weight loss, surgical intervention, and alternative devices like a mouth guard, are also available. With severe sleep apnea, there are only two well tested treatment options: the inspire implant and the CPAP mask. Recently the FDA approved the VIVOS mouthguard for the indication of severe sleep apnea. How this will pan out over time is anybodies guess. In this blog post, we will explore various treatment options for severe sleep apnea, including the use of a mouth guard, to give you a comprehensive understanding of the alternatives.
1. Weight Loss
In cases where obesity is a significant contributing factor to sleep apnea, weight loss can be an effective adjunctive treatment. Obesity is tightly correlated to sleep apnea however the causality pattern is complicated. Losing even a modest amount of weight can help reduce the severity of sleep apnea symptoms. However, even significant weight loss that is maintained over time has been unable to slow the progression of sleep apnea.
Shedding excess pounds can lead to a decrease in the amount of fatty tissue in the throat, reducing obstructions and improving airflow during sleep. More importantly is the idea of the heavy abdomen impinging on the excursion of the diaphragm, making breathing difficult. At TSS we also deal with cellular efficiency and cellular medicine. We have a comprehensive wellness center to help maximize the effect of healthy nutrition on metabolically compromised obese individuals. A combination of regular exercise, a balanced diet, and professional guidance can aid in achieving sustainable weight loss goals.
2. CPAP (Continuous Positive Airway Pressure)
CPAP therapy is mentioned as the gold standard treatment for sleep apnea, especially for severe cases. This is not deserved in my opinion since 50% of patients fail the CPAP in the first year. Most if not all of these patients have an undiagnosed nasal obstruction. If the nose is evaluated and treated this number drops significantly. It involves wearing a mask that is connected to a machine that delivers a continuous flow of pressurized air into the airway, preventing it from collapsing during sleep. CPAP therapy effectively maintains an open airway and ensures an adequate oxygen supply throughout the night. While CPAP can be highly effective, compliance can be a challenge due to discomfort, mask leakage, and perceived social stigma. Bottom Line: Get your nose evaluated and your airway maximized by an ENT before committing to a lifetime of wearing a mask.
3. Inspire Upper Airway Stimulation Therapy (UAS)
Inspire is an innovative implantable device designed for patients with moderate to severe obstructive sleep apnea who cannot tolerate CPAP therapy. It works by delivering electrical impulses to the nerves that control the tongue and other airway muscles, helping to maintain an open breathing passage during sleep. The device is programmed to detect breathing patterns and stimulate the airway muscles to prevent obstructions. Inspire therapy is considered an effective alternative to CPAP for patients who meet certain criteria and can tolerate the surgical implantation process. The ADHERE study has shown Inspire to be more effective than the CPAP mask in virtually every metric we use to evaluate cure.
4. VIVOS Mouth Guard
The VIVOS Oral Appliance is a relatively new alternative to CPAP therapy and surgical options for the indication of SEVERE sleep apnea. It is a custom-made mouth guard designed to reposition the jaw and enlarge the upper airway space. By gently moving the lower jaw forward, the VIVOS mouth guard helps prevent the collapse of the airway during sleep, reducing apneas and improving airflow. Oral appliances like the VIVOS device are usually useful for individuals with mild to moderate sleep apnea who cannot tolerate CPAP therapy or desire a non-invasive treatment option. VIVOS is the first mouthguard to receive the FDA indication for the treatment of SEVERE sleep apnea. It should be noted that although the VIVOS mouth guard is suitable for severe sleep apnea cases, only time will tell if this indeed turns out to be true. A proper assessment by a qualified healthcare professional is necessary to determine the suitability of any treatment.
Choosing the Right Treatment

The choice of treatment for severe sleep apnea ultimately depends on factors such as the severity of the condition, individual preferences, and medical advice. It is crucial to consult with a sleep medicine specialist or a healthcare professional experienced in sleep disorders for an accurate diagnosis and appropriate treatment recommendation. They will consider various factors, including the patient’s medical history, anatomical characteristics, and sleep study results to guide treatment decisions.
Conclusion
Severe sleep apnea requires professional intervention and appropriate treatment to mitigate the associated health risks and improve overall well-being. While continuous positive airway pressure (CPAP) is the most common treatment, alternative options, such as weight loss, the Inspire implant, and the VIVOS mouth guard can be viable alternatives for certain patients. An individualized approach to treatment, based on the severity of sleep apnea and patient preferences, provides the best chance for effectively managing the condition and promoting restful, healthy sleep. Always consult a healthcare professional to find out which treatment option is suitable for your specific needs.